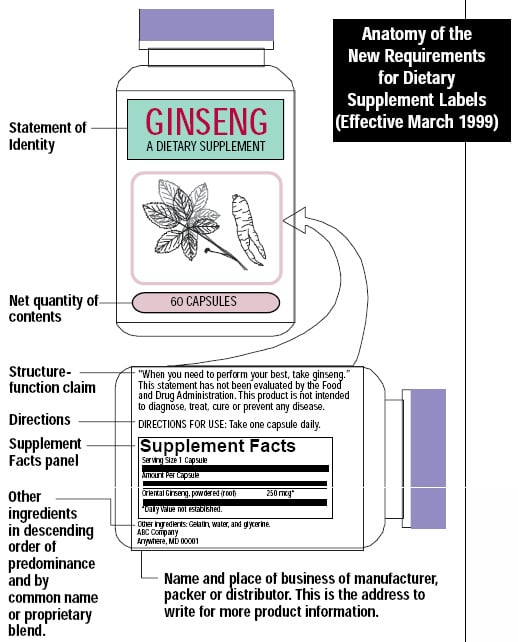
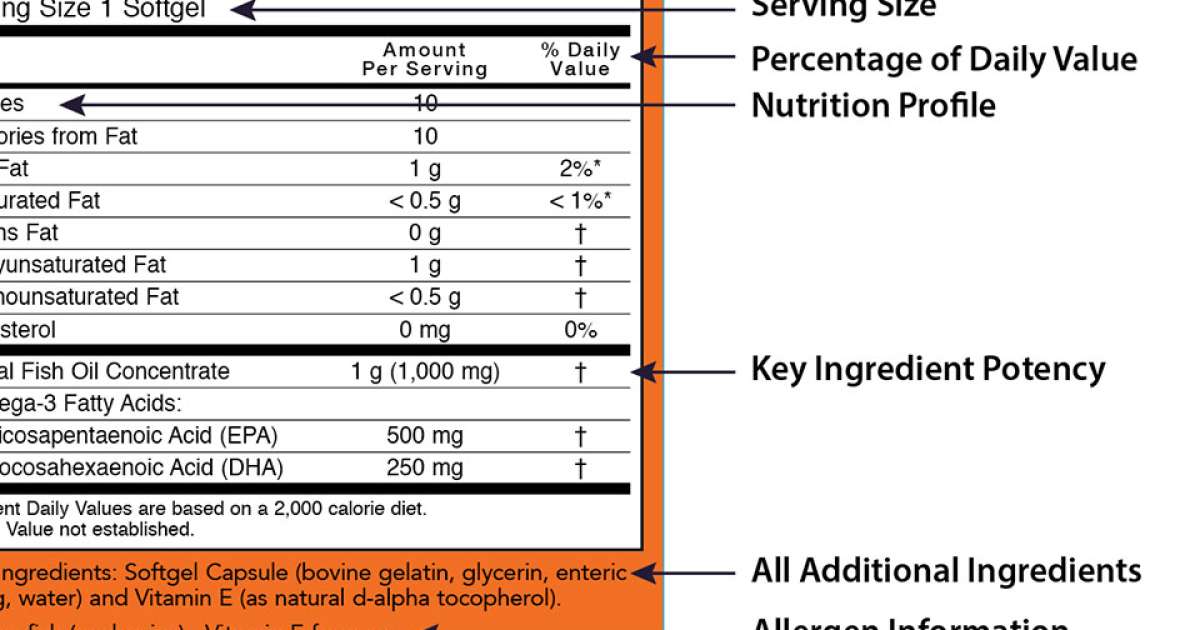
40 structure function claims on dietary supplement labels
EOF FDA Labeling Guidance: Making Structure/Function Claims for Dietary ... the fda permits supplements to so-called "structure-function claims," which are claims that describe the role of a nutrient or dietary supplement "intended to affect the structure or function in...
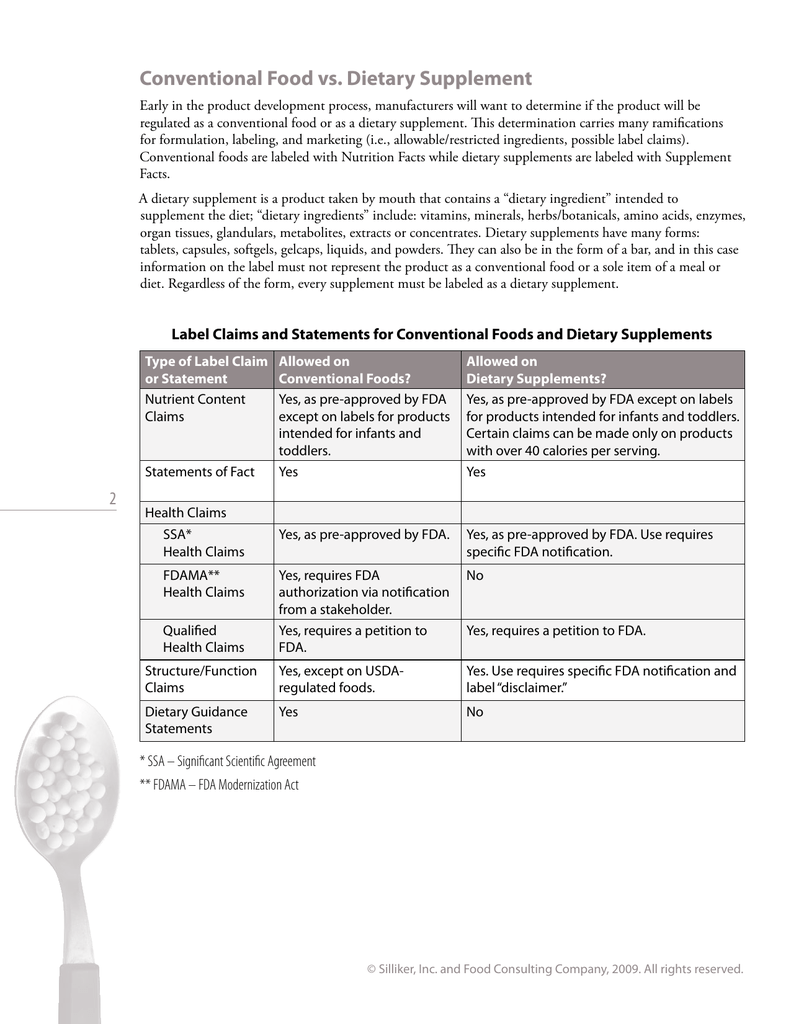
FDA Supplement Guide - innerself.com The 1994 Dietary Supplement Health and Education Act, which set up a new framework for FDA regulation of dietary supplements, requires consumers, as well as manufacturers, to be responsible for checking the safety of these products and determining the truthfulness of their label claims.
Structure function claims on dietary supplement labels
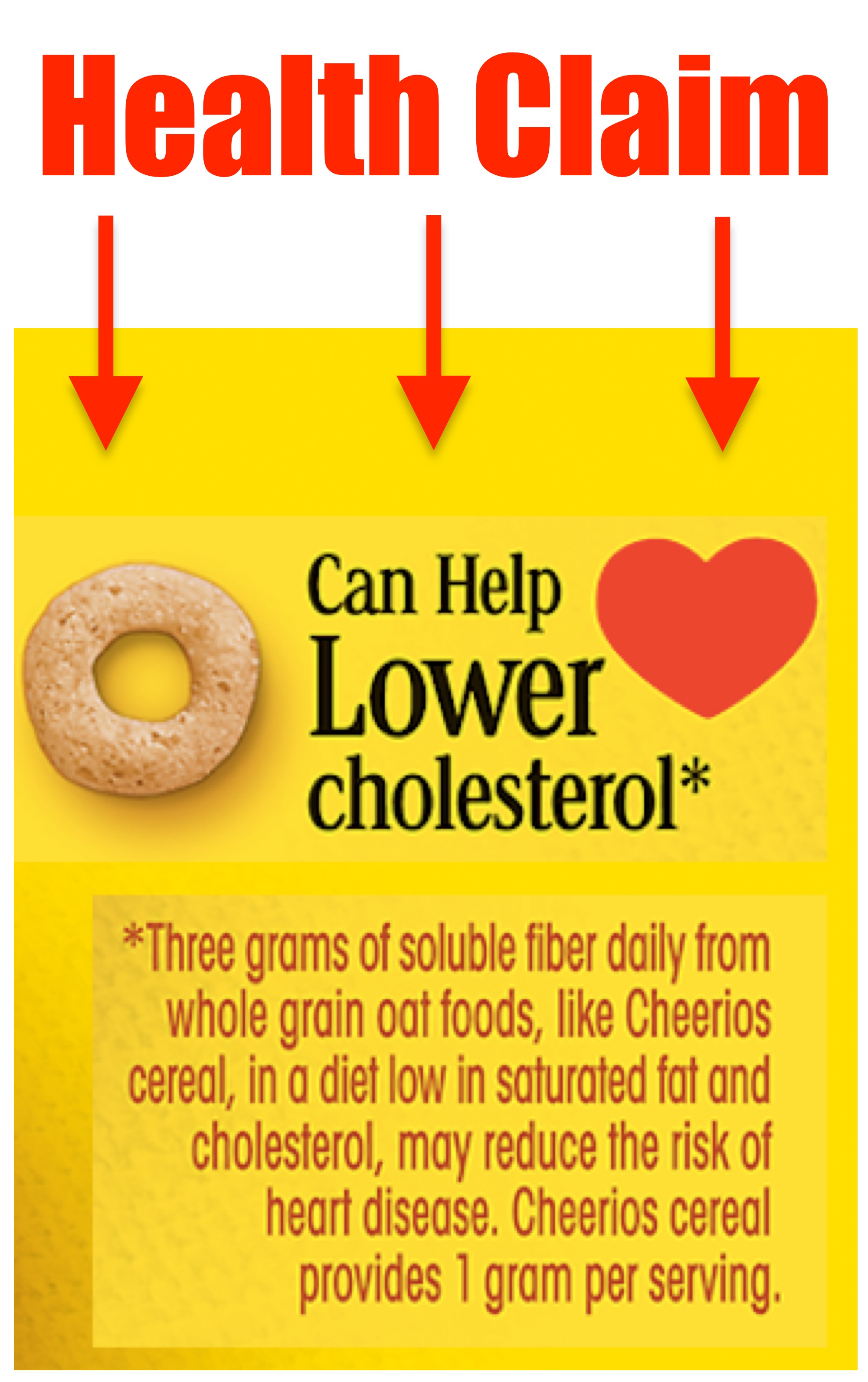
Dietary Supplements: An Advertising Guide for Industry In contrast to health claims, "structure/function" claims, within the broader category of "statements of nutritional support," refer to representations about a dietary supplement's effect on the structure or function of the body for maintenance of good health and nutrition. Structure/function claims are not subject to FDA pre-authorization. Dietary Supplements Claims, Labels and Regulations | NSF Structure/function claims refer to the supplement's effect on the body's structure or function, including its overall effect on a person's well-being. Examples of structure/function claims include "Calcium builds strong bones" and "Antioxidants maintain cell integrity". Nutrient Content Claims Structure/Function Claim Notification for Dietary Supplements Office of Dietary Supplement Programs (HFS-810) Center for Food Safety and Applied Nutrition Food and Drug Administration 5001 Campus Drive College Park, MD 20740-3835 Contact the Office of Dietary...
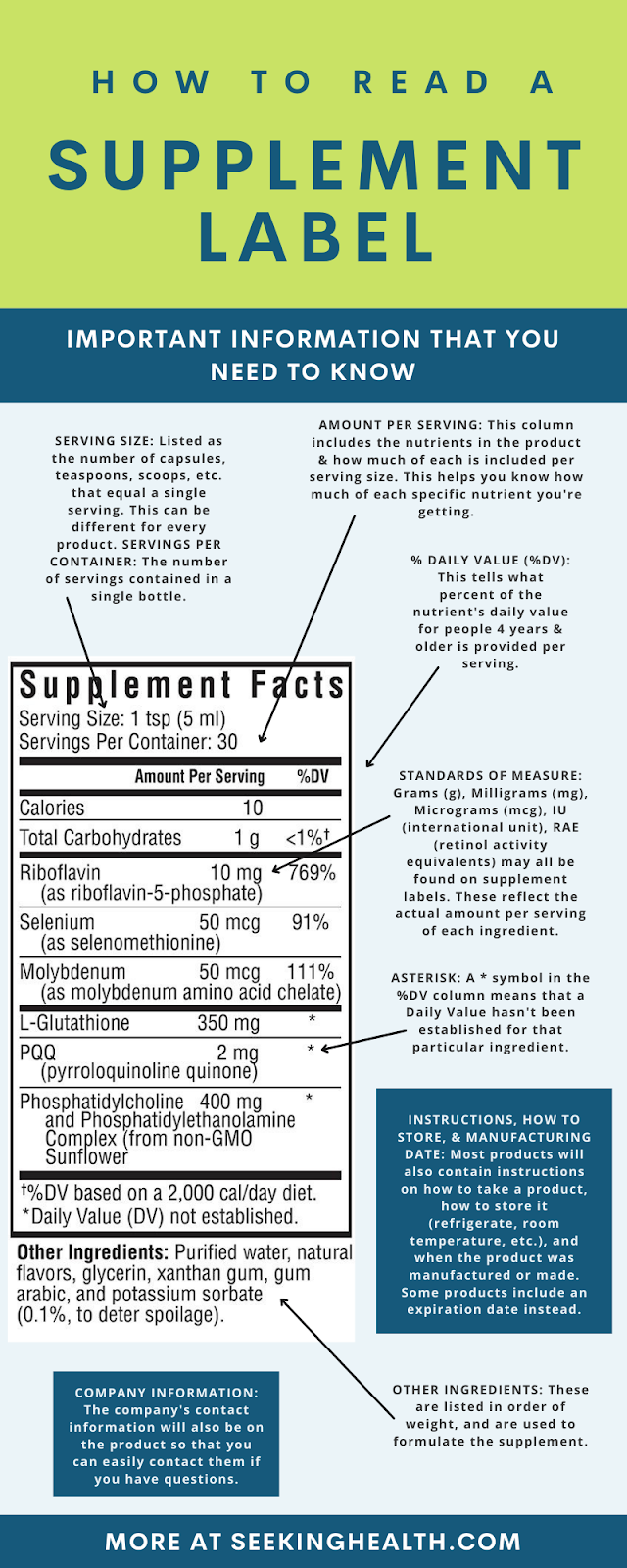
Structure function claims on dietary supplement labels. Structure/Function Claims | FDA The Dietary Supplement Health and Education Act of 1994 (DSHEA) established some special regulatory requirements and procedures for structure/function claims and two related types of dietary... Statements For Dietary Supplements: Structure/Function Claims Review ... The FDA published final regulations defining the types of structure or function claims permitted on dietary supplement labels in 2000, 21 CFR 101.93: Certain Types of Statements for Dietary ... FDA Labeling Guidance: Making Structure/Function Claims for Dietary ... the fda permits supplements to so-called "structure-function claims," which are claims that describe the role of a nutrient or dietary supplement "intended to affect the structure or function in humans" or characterize the "documented mechanism" by which the nutrient acts to maintain such structure or function, but do not claim to "diagnose, … Structure/Function Claim Notification for Dietary Supplements Office of Dietary Supplement Programs (HFS-810) Center for Food Safety and Applied Nutrition Food and Drug Administration 5001 Campus Drive College Park, MD 20740-3835 Contact the Office of Dietary...
Dietary Supplements Claims, Labels and Regulations | NSF Structure/function claims refer to the supplement's effect on the body's structure or function, including its overall effect on a person's well-being. Examples of structure/function claims include "Calcium builds strong bones" and "Antioxidants maintain cell integrity". Nutrient Content Claims Dietary Supplements: An Advertising Guide for Industry In contrast to health claims, "structure/function" claims, within the broader category of "statements of nutritional support," refer to representations about a dietary supplement's effect on the structure or function of the body for maintenance of good health and nutrition. Structure/function claims are not subject to FDA pre-authorization.



























Post a Comment for "40 structure function claims on dietary supplement labels"